Antimicrobial Use and Stewardship
CDFA-AUS • 1220 N Street, Sacramento, CA 95814 • 916-576-0300Antimicrobial Use & Stewardship Program
Program Updates

|
AUS 2024 Annual Report | |

|
2023 Veterinary Feed Directive Summary Report | |

|
AUS Fact Sheets: 2019 NAHMS Goat Study – CA Results | |

|
Antimicrobial Selection: Considerations for Veterinarians |
Since the introduction of antimicrobials in the 1940s, illness and deaths in both people and animals from infectious diseases have been greatly reduced. Unfortunately, as these drugs have been used so widely for so long to treat both humans and animals, many of the germs (bacteria) are adapting and are no longer vulnerable, making them resistant to these wonder drugs.
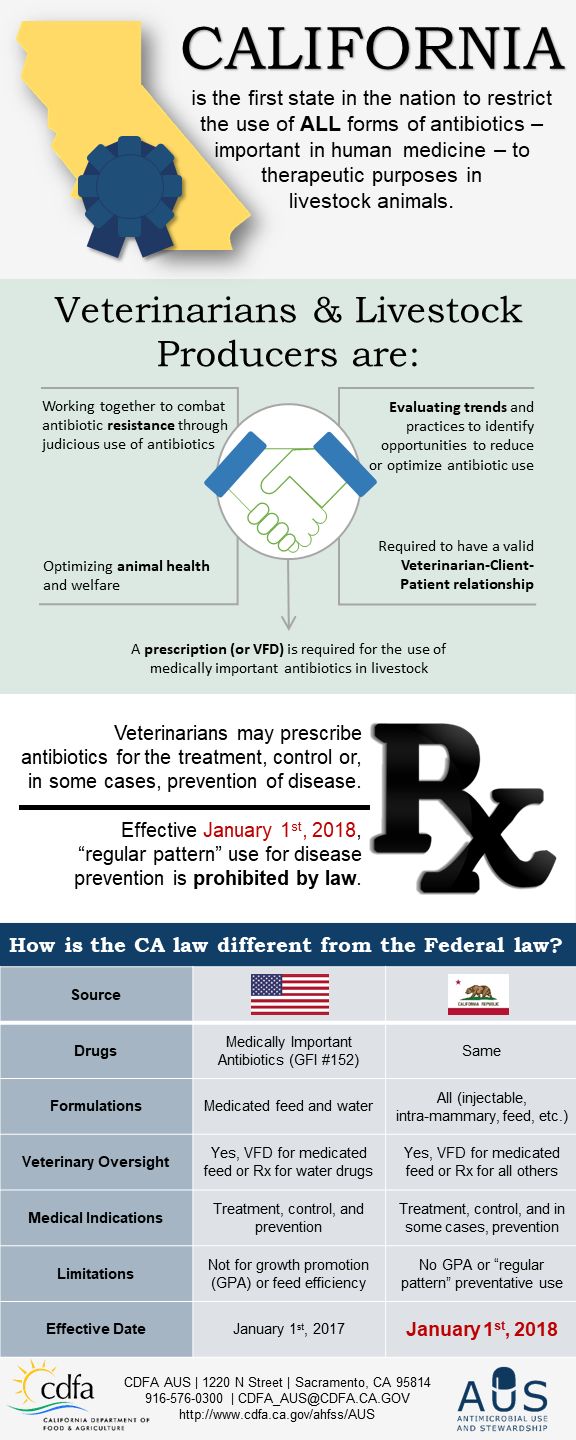
A coordinated effort by physicians, veterinarians, individual patients, animal caretakers and producers is essential in order to preserve the efficacy of antimicrobial drugs. CDFA’s Antimicrobial Use & Stewardship program provides the education and tools for veterinarians and producers to make decisions regarding disease prevention and judicious use of antimicrobials in livestock. The intent is to uphold best animal health practices in order to provide a safe, secure, and bountiful food supply, while reducing the emergence of antimicrobial resistance.