Livestock Drug Program
1220 N Street, Sacramento, CA 95814 • 916-900-5022 • feed_lvstk@cdfa.ca.govThe Livestock Drug Program regulates sales of all over-the-counter livestock drugs in California. The Program reviews and approves product registrations, issues retailer licenses, and conducts inspections to verify compliance. Fees are collected for licensing and registration to fund this program.
Licensing, Registration, and Fees
The Livestock Drug program requires registration for all livestock drug products including restricted livestock drugs being sold within, or into, the state of California. All retail locations must have a Restricted Livestock Drug License to lawfully sell restricted livestock drugs. Failure to obtain the necessary credentials to sell livestock drugs may result in the immediate quarantine of products. For more information on licensing, registration, and fees, click the heading.
General Information
Restricted Livestock Drugs
Additional resources will be posted as they become available.
- Restricted Livestock Drug Frequently Asked Questions (FAQs)
- CDFA Registered Restricted Livestock Drugs
For information about medically important antimicrobial drugs, please visit the Antimicrobial Use and Stewardship website.




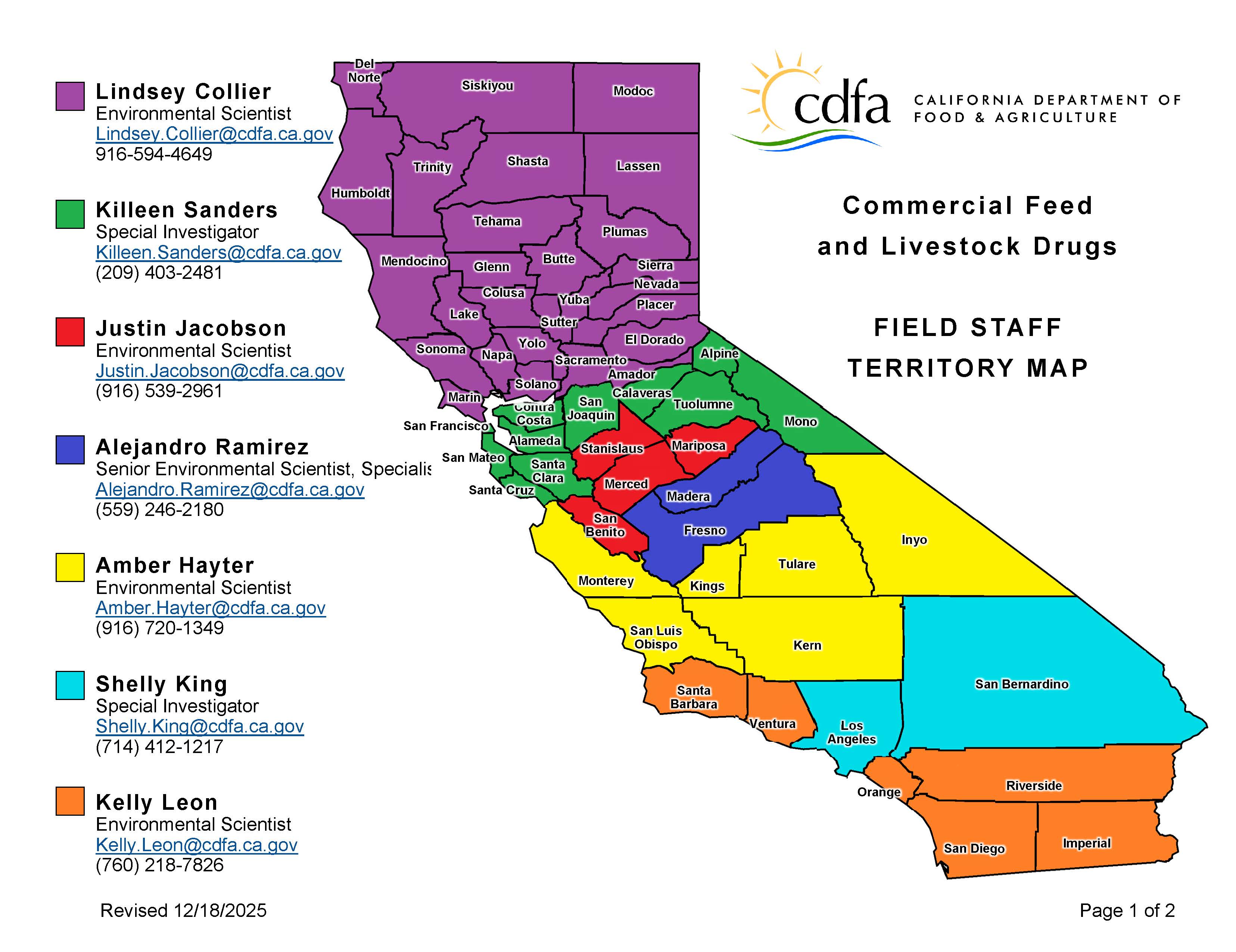
 Commercial Feed Regulatory Program
Commercial Feed Regulatory Program