Preventative Release Program (Medfly)

An environmentally safe and biologically sound approach to the control and eradication of fruit fly infestations in Southern California.
Medfly Preventive Release Program Facility

The Mediterranean Fruit Fly Preventive Release Program (Medfly PRP) located in Los Alamitos, California, is a joint program of the United States Department of Agriculture (USDA) and the California Department of Food and Agriculture (CDFA). The objective of the PRP is to prevent establishment of Medfly colonies in California utilizing a scientific process known as sterile insect technique (SIT). SIT is a biologically-based birth control method involving sustained releases of large numbers of sterile males into a target area to reduce the reproductive potential of wild introductions. Matings between these sterile males and wild females result in the production of infertile eggs. This "birth-control" approach can be used to prevent, and eradicate Medfly populations, with no side-effects on the environment. The PRP also maintains an operational infrastructure to be utilized as needed to respond to Medfly infestations both within and outside the PRP area.
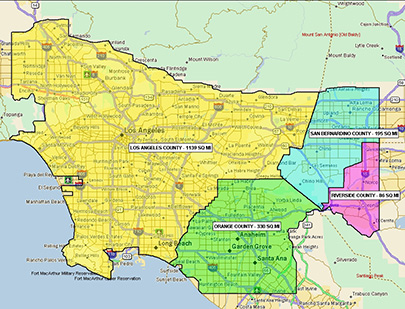
Prior to the establishment of the PRP, California employed a reactive approach to repeated Medfly introductions throughout the state. These eradication efforts were costly and sometimes involved controversial aerial applications of pesticide. In 1996, the Medfly PRP was formed to proactively address the escalating threat of introduction present in the Los Angeles Basin as a result of increased trade and travel. Several changes have been made to the coverage area since program inception, but the Medfly PRP currently encompasses 1,750 square miles and includes portions of Los Angeles, Orange, San Bernardino, and Riverside counties. When infestations do occur, the strategic location of the Medfly PRP facility in Los Alamitos allows for a swift and economical response anywhere in California.
The cost to fund the PRP is approximately 16 million dollars annually with each Department contributing equivalent funding through a cost share agreement. This cost is minimal when compared to the costs of eradication efforts and also serves to limit quarantines imposed upon U.S. growers and industry, thus enhancing global trade efforts. Additionally, no pesticides are utilized in the Medfly PRP, making the program environmentally-friendly.
The Medfly PRP consists of four major components that operate year round. These components involve the release of sterile Medflies at the weekly rate of 62,500 to 125,000 flies per square mile within the coverage area, trapping for the detection of wild Medfly introductions at the rate of five Jackson traps and five McPhail traps per square mile with inspections at weekly intervals, identification of trapped flies by entomologists, and data management and review to monitor the quality and effectiveness of the PRP.
The Medfly PRP has proven to be an effective tool in the battle to keep California free of Medfly. Prior to inception of the PRP, California averaged 7.5 infestations per year. Since preventive releases began, the number of infestations has dropped by over 90%, averaging less than one annually.
The Medfly PRP receives shipments of Medfly in the pupae life stage from a CDFA production laboratory (lab) located in Hawaii and a USDA supported lab located in Guatemala. The breeding colonies are maintained in labs located in areas outside of the Continental United States where Medfly is already established to eliminate the risk of fertile flies escaping the lab and triggering infestations. The PRP receives over 223 million sterile Medfly pupae weekly.
Prior to shipment, pupae are mixed with a fluorescent pink or orange external marker dye that will transfer to the adults upon emergence from the pupal casing. The dye which fluoresces when exposed to ultraviolet (UV) light is used to identify the flies as sterile, differentiating them from wild flies. After mixing with the dye, the pupae are poured into tubular plastic bags and any residual air in the bag is pressed out prior to sealing. The pupae are shipped in a state of hypoxia to slow the flies’ metabolism and prevent damage during shipment. A dosimeter tag is attached to each bag and the bags are placed one-by-one into an irradiator where they are exposed to 15 Krad, 150 Gray gamma radiation for several minutes. The dosimeter tag will indicate whether or not the flies received a sufficient dose of radiation to render them sterile. After the pupae are removed from the irradiator, the dosimeter tags are inspected to ensure sterility and the flies are placed in boxes for shipment to the PRP facility. Ice packs are added to the boxes in order to maintain a temperature between 62-66 degrees Fahrenheit while in transit.

Upon emergence from the pupal casing, the adult flies will require food and water. In order to meet this need, each day two batches of diet are prepared in commercial kettles within the diet preparation room. Each batch of the diet formulation is composed of 50 gallons of water, 73 pounds of sugar, 3 pounds of agar, and 0.04 pounds of methyl paraben preservative. The ingredients are combined in the kettles and brought to a boil. The hot solution is then dispensed into stacking trays to cool, congealing in the process. The following morning, the diet is cut into blocks and transported to the pupae preparation rooms. Combined, the two batches of diet are sufficient to feed 35 million flies.

Once the boxes of pupae arrive at the PRP facility, they are transported to a pupae preparation room and opened in the order in which they were packed. As the boxes are opened, each bag of pupae is inspected for evidence of proper irradiation and a thermometer is inserted into the center bag to obtain a temperature reading. The bags are opened and the pupae are poured into a bucket from which a representative sample from each bag is obtained and submitted to Quality Control for testing.

The pupae are then poured into a volumizer that meters out the desired amount of pupae into a specially engineered pyramid dispenser. As empty incubation trays are fed through the dispenser, the volumizer is activated and 350 milliliters of pupae are released into the pyramid dispenser that evenly distributes the pupae into a channel around the perimeter of each incubation tray. After trays exit the dispenser, two pieces of diet are placed on opposing corners of the trays. The trays are then stacked on a cart until the total pupae in the stack reaches one million at which point a circulation fan is placed on top of the stack. This mobile stack of incubation trays is referred to as a “tower.” Tower heights vary depending upon pupae size but most towers are composed of between 42 to 56 trays. On average, 35 towers are produced each day.


Upon completion, each tower is transported to an incubation room where the circulation fan is plugged in. Orange flags attached to each fan allowing for a quick visual inspection to determine if all the fans in the room are operational. If a fan stops rotating, humidity increases within the tower to a level at which the flies will drown. Each tower is labeled with the shipping, prep and release dates; the lab of origin; and an individual identification number that reflects the order in which the tower was built in relation to the other towers. Environmental controls in the incubation rooms are set at 78 degrees Fahrenheit and 65 percent relative humidity. HVAC units, humidifiers, dehumidifiers, fans, and environmental sensors are employed to achieve the desired environment. Lights are turned off during incubation to limit fly activity. Most flies emerge during the first 48 hours and have been an adult for 2 to 3 days by harvest time. After emergence, the flies migrate from perimeter channel toward the center of the tray where they feed on the diet.

Female Medflies are known to display a high degree of mate discrimination. Test results have shown that exposure to the aroma of ginger during incubation enhances sterile male’s mating competitiveness against wild males. As a result of this testing, a cotton wick containing 1 ml of ginger root oil is placed underneath each tower 24 hours prior to release. The tower circulation fan draws the scent past the flies and throughout the room. This process improves the effectiveness of the release increasing the odds that the sterile males will successfully out-compete wild males for the opportunity to mate with any wild female introductions.

Incubation rooms are frequently inspected throughout the process to ensure the parameters are adequate and all equipment is functioning.
After four days of incubation, a majority of the flies have emerged from the pupal casings and reached peak sexual maturity. The eclosion towers containing the flies are removed from the emergence rooms and transported to cooling rooms that have been pre-chilled to 38° Fahrenheit. Exposure to the cold air slows the flies’ metabolism rendering them inactive and enabling processing. The number of towers included in each load varies from 5 to 13 depending upon various factors including yield and application rate. A proportional mixture of flies from each laboratory of origin is included in each load in an effort to achieve an even distribution of fly quality throughout the release area. After approximately 45 minutes of exposure to the cold, the flies are ready for processing. The diet is removed by hand from each eclosion tray and the empty pupal casings are extracted using a specially engineered vacuum. Each tray is then inverted over a collection funnel causing the flies to drop into collection trays underneath. Samples are taken from towers of each laboratory and provided to the Quality Control department for post-processing flight-ability testing.

- After all of the flies have been processed, they are poured into a release box and weighed by laboratory origin. Depending upon the number of towers used, loads consist of 45 to 130 pounds equating to 3 to 9 million flies.

The release boxes containing the chilled flies are transported to the nearby airfield and loaded onto the release aircraft. Mounted in the cargo area of each aircraft is an insect release machine that both maintains the chilled state of the flies and dispenses them at the prescribed release rate. Each aircraft has been modified to accommodate the release machine including the installation of two release chutes that protrude from underneath the aircraft's fuselage.

Releases are conducted by a private release operator under contract with the USDA. The current contractor utilizes and maintains five twin-engine turboprop Beechcraft "King Air" aircraft to accomplish the releases. Each aircraft is staffed with a two-person crew. The pilot operates the aircraft and the copilot operates and monitors the release machine and records the flight data. A typical day consists of four flights conducted by two aircraft. Flight times vary from 2½ to 3 hours depending upon a number of factors including the size of the load, the release rate, and the proximity of the release area to the base of operations. Releases are conducted seven days-a-week, with the exception of periods when the airfield is closed or when weather conditions threaten crew safety or the quality of the release.
The 1,750 square mile program area is divided into 26 contiguous regions of varied size and shape depending upon terrain, airspace restrictions, and flight paths. The majority of the program area has a weekly target application rate of 62,500 flies per square mile. A 243 square mile area in central Los Angeles County has been designated as having a high-risk for introduction based upon historical data and as such, this area has a weekly target application rate of 125,000 flies per square mile. Regions are flown every 3 or 4 days in an effort to ensure that a viable sterile male Medfly population is always present in the field. On average 28 flights per week are required to complete the entire 1,750 square mile release area. During releases, the aircraft fly along predetermined passes, spaced ¼ mile apart, while traveling at a speed of 140 knots (160 mph).

Release altitudes vary by region and depend upon a number of factors including topography and airspace restrictions but the average release altitude is 2,000’ above the ground. At that altitude the flies will disperse evenly throughout the coverage area prior to reaching the ground. The aircraft fly along pre-established release lines guided by a satellite navigation system. A load of flies typically covers an area of 60-65 square miles.
The navigation system provides a map display of each assigned release region. The map allows the crew to know when and where to start and stop releases, as well as, what flight lines to follow. The navigation system tracks the position, air speed, and altitude of the aircraft; status of the release machine; and the speed of release.
After all of the day’s flights have been completed, the release contractor submits the flight data to program personnel for review. Program personnel utilize the data to ensure program quality and to prepare the following day’s assignments.
The onsite quality control lab conducts various tests to evaluate fly quality in both the pupae and adult stages ensuring program effectiveness. These tests include determining the percent emergence, percent fliers, longevity, and sex ratio of flies for each laboratory of origin. Representative composite samples of pupae and adults from each lab are utilized in the tests. Most tests involve 5 replications of 100 flies each. Abnormal, non-flier, and dead flies are counted and subtracted from 100 to obtain percentages of normal, flier, and surviving flies to be compared with established specifications. Environmental controls within the lab are set at 78 degrees Fahrenheit, 65 percent humidity, and a day/night cycle of 14/10 hours to replicate conditions present in the incubation rooms.

The percent emergence test is conducted to determine what percentage of flies emerges from the pupal casings and in what timeframe. The test involves setting up two grids of 100 cells each with a single pupae placed in each cell. A piece of plexi-glass is then placed over the open side of grid and secured with a rubber band. A wire screen attached to the opposite side allows for air circulation within the cells. Grids are placed in both the quality control lab and in the incubation rooms to account for any variance in environmental conditions between the locations. The grids are inspected twice daily. On each inspection, the emerged flies are counted and the percentage recorded.
The percent fliers test is conducted on both pupae incubated in the lab and adult flies collected post-processing to determine what percentage of flies are capable of flight. Testing conducted on adults post-processing is more reflective of the quality of flies released into the environment after having endured the stress associated with incubation, chilling, and processing. To set up the test on pupae, 100 pupae are placed inside each of five plastic tubes that have been dusted with talcum power. The talcum powder creates a slippery surface preventing the emerged flies from walking out of the tubes. The tubes containing the pupae are then placed in a clear plexi-glass cage with screened openings allowing for air circulation within the cage. The cage is then moved to an environmentally controlled room where the temperature is maintained at 78 degrees Fahrenheit, 65 percent humidity, and a day/night cycle of 14/10 hours where they remain for four days. Each morning and afternoon, the flies that have emerged and flown out of the tubes are vacuumed from the cages and counted. On the morning of the fourth day, the contents of the five tubes are counted for unemerged pupae, partially emerged pupae, deformed flies, and normal-appearing flies that were unable to fly out of the tubes. From the original sample of 500 pupae from each shipment, a percentage of emergence and flight ability is obtained.

The sex ratio test is a measure of the ratio of male flies to female flies to verify that the percentage of males does not deviate from the standard of 99% or higher for the “Temperature Sensitive Lethal” (TSL) strain of Medflies. The TSL strain of flies has been developed to enable elimination of virtually all of the females while in the egg stage, resulting in 99 to 100% of adults being male. The use of all-male strain of sterile flies has benefits over the use of a 50/50 male/female strain in that it eliminates sterile females as mates of the sterile males. This causes the sterile males to more aggressively seek out mates, and because the flies are all male, resources aren’t wasted on female production and release.
On occasion, a longevity stress test is performed to obtain a measure of the nutrient reserves of the flies. Shortly after emergence, 100 flies are placed in a cage without food, water, or light where they remain for 48 hours. The dead flies are counted and the percentage survival is obtained. A survival rate of 50 percent is considered acceptable.
Traps engineered to catch male and female Medflies are placed throughout the State and are checked weekly. The contents of the traps are removed and sent to the PRP identification lab for inspection. Personnel look for Medflies absent of dye and for any other fruit flies that may pose a threat to California Agriculture.
Types of Traps

If a Medfly is examined and shows no evidence of the fluorescent dye, a trained insect biosystematist determines the sterility through the dissection and examination of the reproductive organs of the fly. A wild Medfly is indicative of a possible infestation.


